Abstract
Background: Tagraxofusp is a protein-drug conjugate consisting of a human interleukin-3 fused to a truncated diphtheria toxin payload and is the first CD123 targeted agent approved by the FDA. Tagraxofusp is approved as a single agent for the treatment of individuals age 2 years and above with Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) based on a 90% overall response rate and favorable toxicity profile (Pemmaraju et al. NEJM 2019; 380:1628-1637). CD123 is widely expressed on a variety of other hematologic malignancies, including the majority of patients with acute myeloid leukemia (AML) and some subtypes of B- and T-cell acute lymphoblastic leukemia (ALL). The potent activity of tagraxofusp in BPDCN, coupled with the favorable toxicity profile, makes tagraxofusp a compelling agent for study in other CD123-expressing malignancies with high unmet needs. Our proposal to study this agent represents the first trial of tagraxofusp as a single agent and in combination with other agents for children and young adults with CD123-expressing hematologic malignancies.
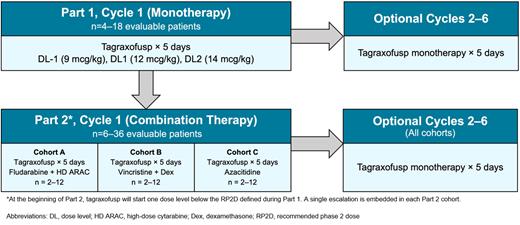
Study Design and Methods: This is a non-randomized, open-label, multicenter phase I dose-determination trial for children < 21 years of age with relapsed and/or refractory CD123-expressing hematologic malignancies (NCT05476770). The study is conducted through the Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) Consortium and consists of 2 sequential parts. Tagraxofusp is administered intravenously once daily over 5 days for both parts. In Part 1, tagraxofusp monotherapy is initiated at the FDA-approved dose of 12 mcg/kg and incorporates a single dose escalation and de-escalation. In Part 2, tagraxofusp will be initiated at one dose level below the Part 1 recommended dose in combination with other agents in three cohorts: Cohort A incorporates myeloid-directed combination therapy (fludarabine, high-dose cytarabine); Cohort B incorporates lymphoid-directed combination therapy (vincristine, dexamethasone); and Cohort C incorporates the hypomethylating agent azacitidine. Each cohort includes a single dose escalation of tagraxofusp. Cohort allocation is determined by investigator preference but patients with measurable residual disease (defined as 1% to < 5%) are only eligible for Cohort C. Depending on response, patients in both parts and all cohorts are eligible to receive subsequent cycles of tagraxofusp monotherapy (maximum of 5 additional cycles).
Key Eligibility: Patients age 1 to 21 years with two or more relapses or refractory CD123-expressing hematologic malignancies are eligible for Part 1 and patients with hematologic malignancies in first relapse or refractory to de novotreatment are eligible for Part 2. Patients with Down Syndrome are eligible to receive tagraxofusp as monotherapy at the same dose level as patients with non-Down Syndrome. Patients with BPDCN in first relapse are eligible for both parts. Confirmation of qualitative CD123 positivity at the treating site is required.
Objectives: The primary objectives are to assess safety and tolerability of tagraxofusp monotherapy (Part 1) and in combination with chemotherapy (Part 2) in pediatric and young adult patients with relapsed/refractory CD123-expressing hematologic malignancies. Secondary objectives include pharmacokinetics and anti-tumor activity within the context of a phase I study. Correlative studies will assess for biomarkers of tagraxofusp response and explore potential mechanisms of resistance.
Sample Size and Statistical Design: Part 1 utilizes a standard 3+3 dose escalation/de-escalation design and, based on safety and tolerability, is planned to enroll between 4 and 18 patients with 3 dose levels. Part 2 also utilizes astandard 3+3 design but with a single escalation and is planned to enroll between 6 and 36 patients.
The study is open to accrual at select TACL sites.
Support: This study is conducted by the TACL Consortium with financial support from Stemline Therapeutics.
Disclosures
Brooks:Stemline Therapeutics: Current Employment. Mughal:Stemline Therapeutics: Current Employment, Current holder of stock options in a privately-held company; Oxford University Press: Other: financial benefit and/or patents; Informa: Other: financial benefit and/or patents. Wayne:Kite, a Gilead company: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal